Human amniotic membrane forms the innermost layer of placental tissue. The avascular membrane acts as a protective barrier for the developing fetus. Its properties provide a wide variety of potential benefits in regenerative medicine.

Quality Manufacturing
Our allograft membrane products are processed in compliance with all current Good Tissue Practices as mandated by the United States Food and Drug Administration and the American Association of Tissue Banks. We pride ourselves on quality standards and testing that exceed industry standards.
Our Process
1. Screening
Extensive donor screening to ensure donor suitability.
2. Birth
All tissue collected from caesarean section births to ensure quality and safety.
3. Acquisition
Tissue is delivered and processing is initiated within 24 hours.
4. Processing
Tissue is cleaned and processed using proprietary methods.
5. Sterilization
Essano A is terminally sterilized for additional level of safety.
6. Storage
Essano A is packaged to be stored at ambient temperature.
7. Shipping
Priority shipping with storage instructions.
8. Application
Preparation instructions and records portal access included for HCT/P tracking.
Why Esano A
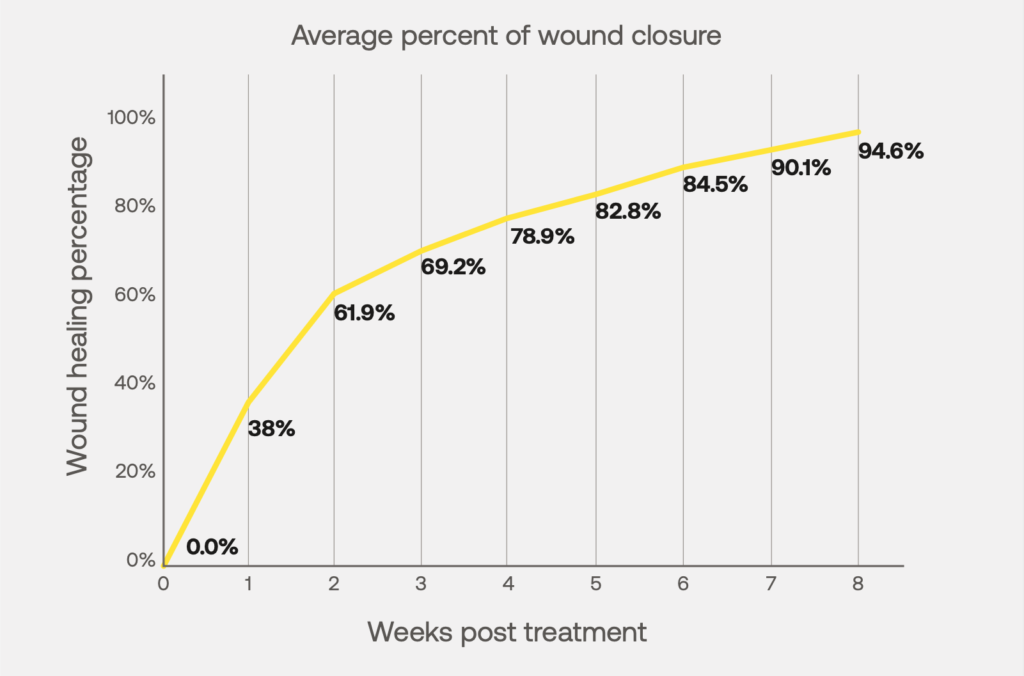
Esano A has been shown to be effective in treating chronic non-healing foot ulcers, including diabetic, pressure, and venous ulcers.
In a 10 patient case series, 95% of wound closure was achieved by week 8 of treatment in patients that previously failed standard of care.